Malt intended for use in beer brewing or elsewhere in the food industry
The aliquot of an extract of malt is added to a buffered starch solution and allowed to stand for exactly 30 min at 20 °C. Then, the maltose – formed primarily from the starch through the action of the β-amylase – is measured using iodine and is determined according to the following chemical reaction:
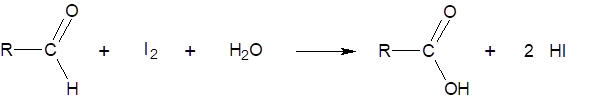
The method describes how to determine the free and total chlorine content using a titrimetric method with DPD.
The analysis involves a reaction with N,N-diethyl-1,4-phenylenediamine (DPD), which forms a compound possessing a red color at a pH of 6.2–6.5. The solution is titrated with an ammonium iron(II) sulfate standard solution until the red color disappears. Total chlorine is measured through the addition of potassium iodide, of which a known amount in excess of that required is added to the solution in advance.
Determination of the concentration of dissolved carbon dioxide in carbonated beverages through titrimetry (dimensional analysis)
This method is suitable for determining the dissolved carbon dioxide in carbonated beverages for concentrations ranging from 0 to 8.4 g/l.
Through the addition of a sodium hydroxide solution, the carbon dioxide in beer becomes bound as sodium hydrogen carbonate or sodium carbonate. Sulfuric acid is added to an aliquot of the beer treated with sodium hydroxide. This causes the carbon dioxide to be released again, after which a stream of air conducts the carbon dioxide into a barium hydroxide solution. Through titration of the excess barium hydroxide, the carbon dioxide content of the beer can be determined [1].
Determination of chlorine dioxide in disinfectants.
Suitable for all solutions containing chlorine dioxide (ClO2).
For the determination of low concentrations (up to 2.5 mg/l) of chlorine dioxide (CIO2) and chlorite the photometric or colorimetric DPD methods are well established. Higher concentrations of ClO2 that are present in the concentrates have to be determined by titration with sodium thiosulphate or after strong dilution.
Chlorine dioxide oxidizes iodide to iodine, which is then reduced by sodium thiosulphate. The sodium thiosulphate consumed is recalculated as ClO2.
|
2 ClO2 + 2 NaI |
→ |
I2 + 2 NaClO2 |
|
I2 + 2 Na2S2O3 |
→ |
2 NaI + Na2S4O6 |